
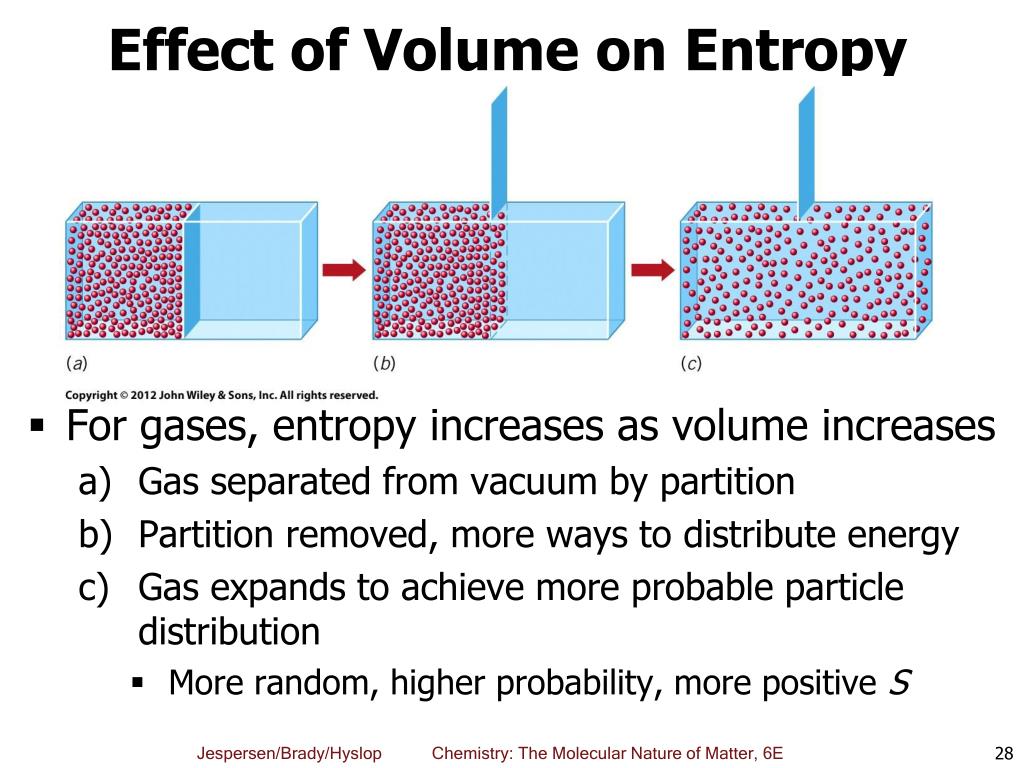
The vibrational frequencies of alicyclic ring systems, J. Thus, we end up with a difference in heat capacities because we conceptually take a trip to $T_2$ as one phase and come back as another phase. , The heat capacity and entropy, heats of transition, fusion and vaporization and the vapor pressures of cyclohexane. The problem statement should make this clear, as other answers are possible if one assumes that the surroundings are adjusted such that vaporization is also reversible at $T_2$, for example.) of vaporization, vap H o: 44.0 kJ/mol Enthalpy change of vaporization at 373.15 K, vap H: 40.68 kJ/mol Std entropy change of vaporization, vap S o: 118.
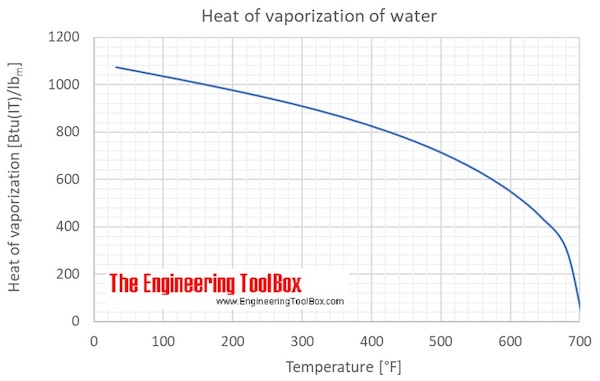
(Edit to address an ambiguity pointed out by Chet: Since I know what relation you're trying to achieve, I know that you mean that $T_1$ is the boiling temperature at the given conditions, i.e., that vaporization is reversible at $T_1$ but not at $T_2\neq T_1$.


 0 kommentar(er)
0 kommentar(er)
